1 a) first start with the forms of asp: pKa's: 2.0 3.9 10.0 H3A+ <====>H2A<====> HA-<=======>A2- 12.0
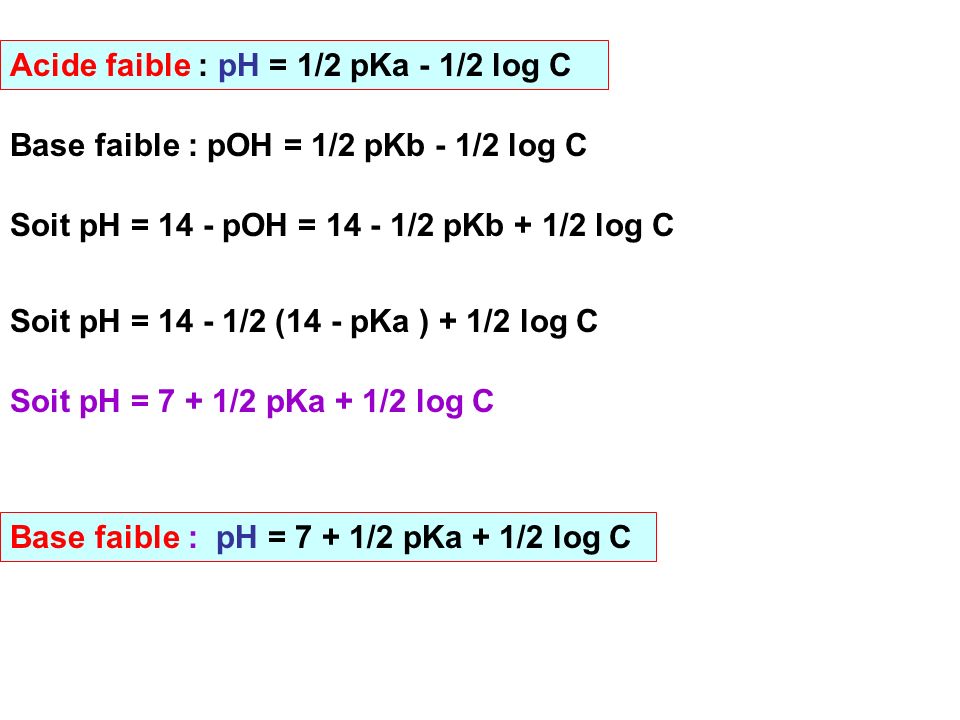
![How is pH = 1/2[pKa - logc] - Chemistry - Chemical and Ionic Equilibrium - 13113047 | Meritnation.com How is pH = 1/2[pKa - logc] - Chemistry - Chemical and Ionic Equilibrium - 13113047 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/ck-files/ck_57fe3e2aeb864.png)
How is pH = 1/2[pKa - logc] - Chemistry - Chemical and Ionic Equilibrium - 13113047 | Meritnation.com
Page 1 of 7 Chem 201 Lecture11 Summer'07 Admin: recall all Test #1's Please turn in Test 1 for regrading Last time: 1. calib

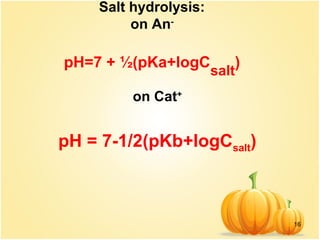
Match the List - I (solution of salts) with List - II (pH of the solution) and select the correct answer using the codes given below the lists:List - IList - IIA.

PPT - 2- Weak acid - Strong base titration :- eg. CH 3 COOH (pKa = 4.74) and NaOH PowerPoint Presentation - ID:2976551
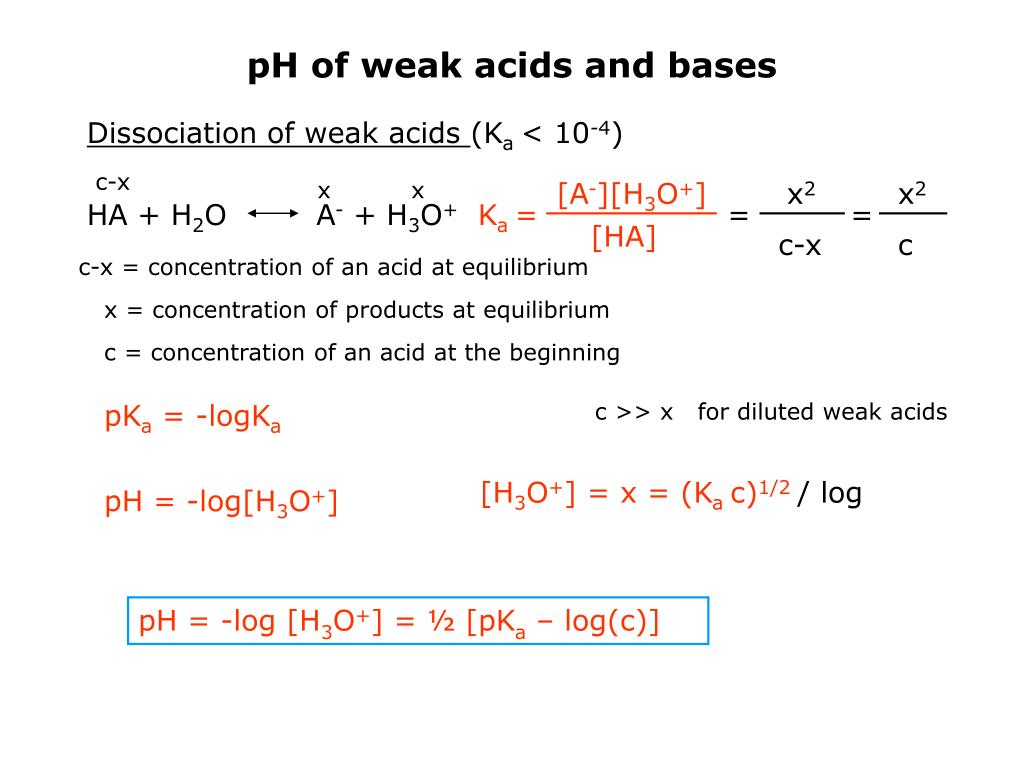
![SOLVED: Equations: pH = pKa log ([b] /[a] (Ka) (Kb) = 1x 10-14 Kb = x2/ (y-x) K = xl/ly x) pH (pKa1 pKa2)/2 pK,'s of amino acid side chains: D (3.9), SOLVED: Equations: pH = pKa log ([b] /[a] (Ka) (Kb) = 1x 10-14 Kb = x2/ (y-x) K = xl/ly x) pH (pKa1 pKa2)/2 pK,'s of amino acid side chains: D (3.9),](https://cdn.numerade.com/ask_images/2531a2f0c6754ab3ba0caf17c487c22e.jpg)
SOLVED: Equations: pH = pKa log ([b] /[a] (Ka) (Kb) = 1x 10-14 Kb = x2/ (y-x) K = xl/ly x) pH (pKa1 pKa2)/2 pK,'s of amino acid side chains: D (3.9),
![Column -I" , "Column -II") ,( (A)" pH of a basic buffer mixture ", (P) pKa+ log"" (["salt"])/(["Acid"])),((B) "pH of an acidic buffer mixture ",(Q) (pKa )(C,Acid) +log""(["Base"])/(["salt"])),((C) "pH of salt solution of Column -I" , "Column -II") ,( (A)" pH of a basic buffer mixture ", (P) pKa+ log"" (["salt"])/(["Acid"])),((B) "pH of an acidic buffer mixture ",(Q) (pKa )(C,Acid) +log""(["Base"])/(["salt"])),((C) "pH of salt solution of](https://d10lpgp6xz60nq.cloudfront.net/question-thumbnail/en_357197801.png)