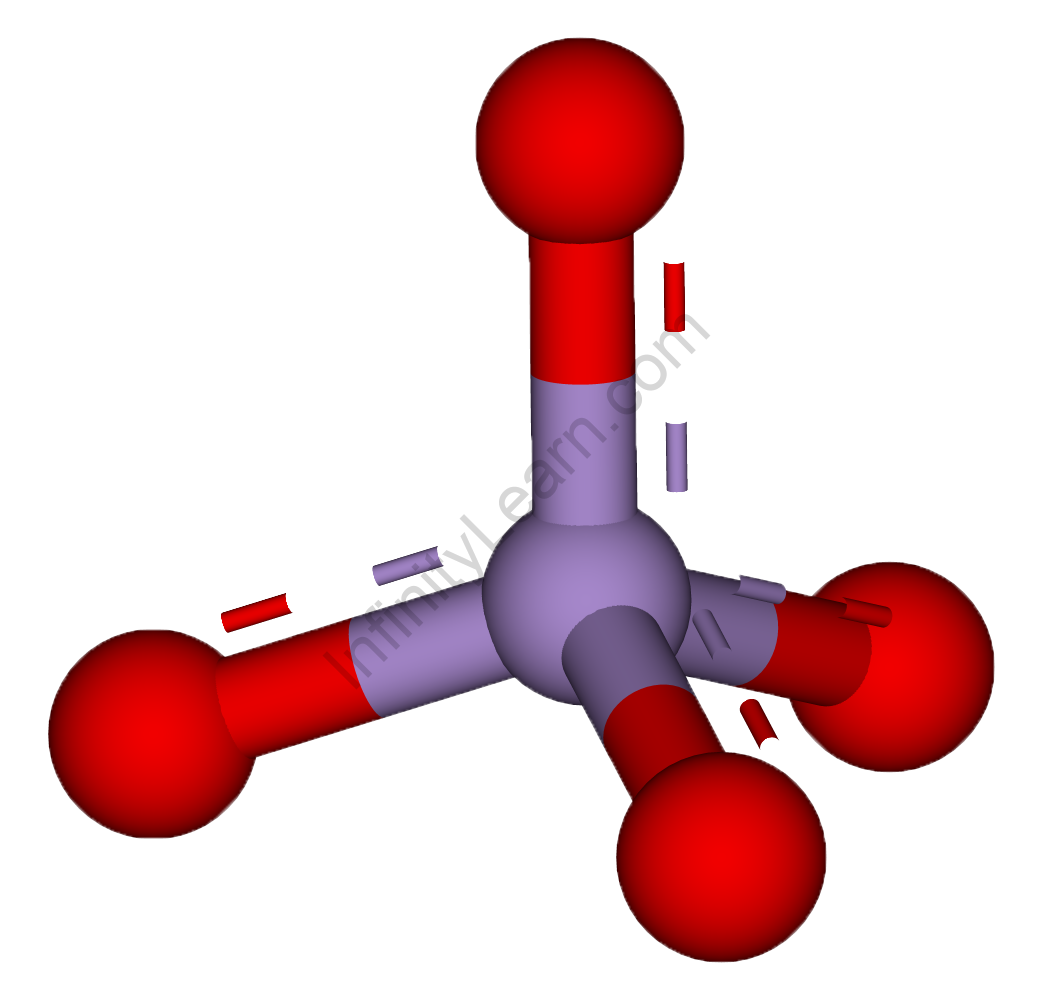
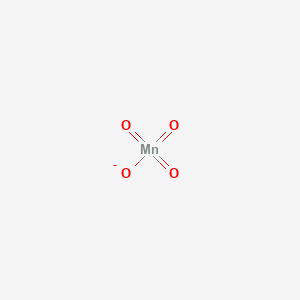
Permanganate Anion, Chemical Structure. 3D Rendering. Atoms are Represented As Spheres with Conventional Color Coding: Manganese Stock Illustration - Illustration of oxygen, model: 188435872
Which one of the following ions exhibits d-d transition and paramagnetism as well? MnO4^-, MnO4^2-, Cr2O7^2-, CrO4^2- - CHEMISTRY FOR NEET

If S + O2→ SO2, H = - 298.2 kJ mole^-1 SO2 + 1/2 O2→ SO3, H = - 98.7 kJ mole^-1 SO3 + H2O → H2SO4, H = - 130.2 kJ
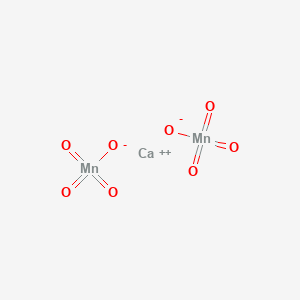
Solved] Give the name of Ca(MnO4)2 and calculate its molar mass. 2. What is the volume, in nm3 , of a wooden block with a volume of 43.7 cm3? 3. Wha... | Course Hero

How hydrogen‐bonded MnO4‐ can influence oxidation of olefins in both gas phase and solution? - Javan - 2012 - Journal of Physical Organic Chemistry - Wiley Online Library
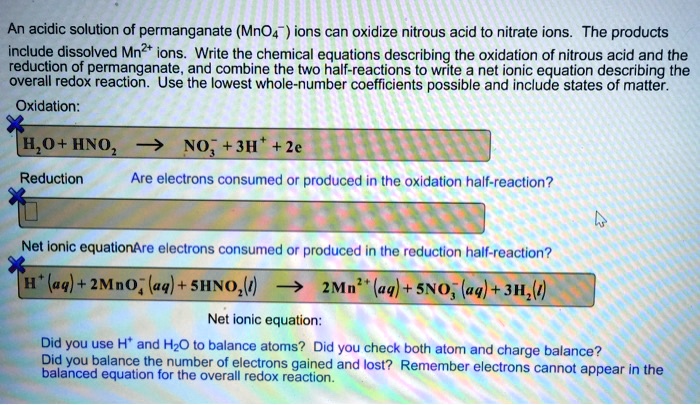
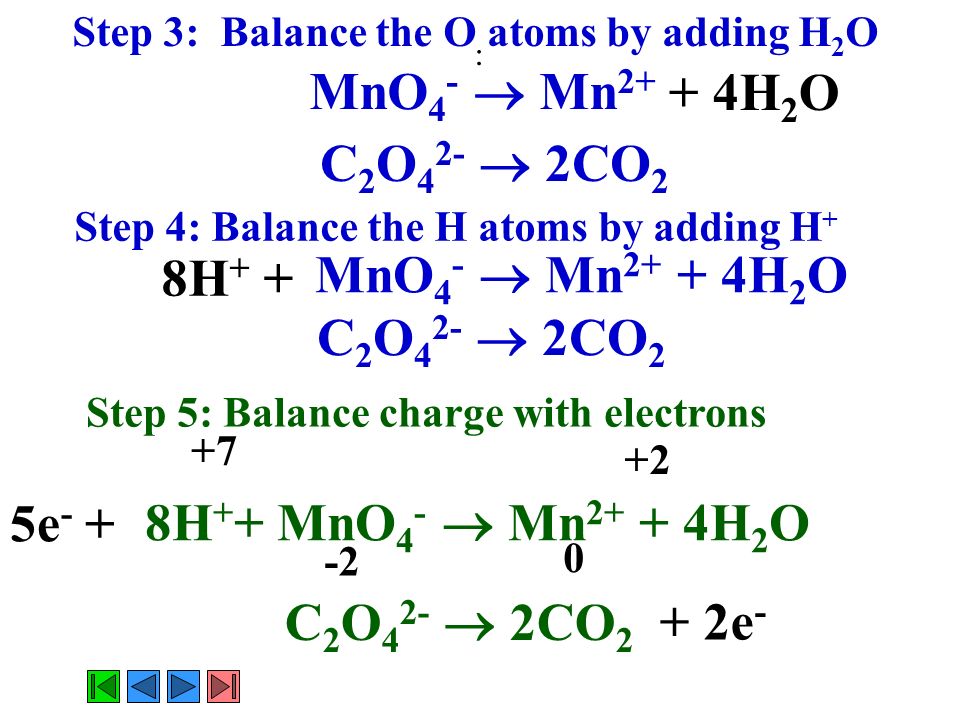
SOLVED:An acidic solution of permanganate (MnO4" ions can oxidize nitrous acid t0 nitrate ions_ The products include dissolved Mn?+ ions Write the chemical equations describing the oxidation of nitrous acid and the

1a) Calculate the oxidation number for one atom of Mn in the KMnO4 using the reaction:... - HomeworkLib
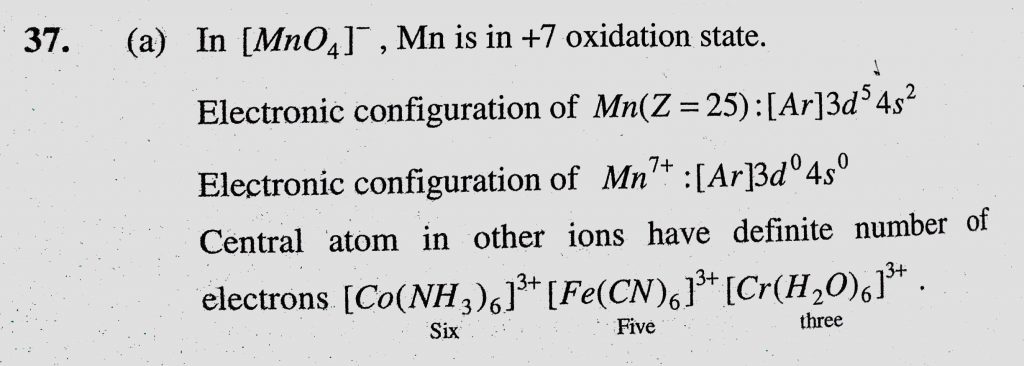
The complex ion which has no 'd' electrons in the central metal atom is (a) (MnO4)- (b) (Co(NH3)6) 3+ - Sahay Sir


.PNG)








.PNG)